In a landmark decision on July 8, 2025, Swiss medicines regulator Swissmedic approved Coartem Baby (Riamet Baby); the globe's first malaria drug specifically developed for newborn babies and infants weighing under 4.5 kg. Coartem Baby was developed by Novartis, together with the Medicines for Malaria Venture (MMV), to bridge a long-standing gap in treatment and potentially save the lives of the vulnerable in sub‑Saharan Africa.
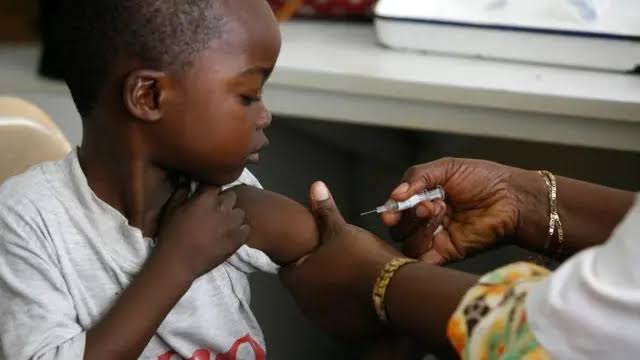
Why This Matters: A Critical Treatment Gap Closed To date, there is no approved antimalarial treatment for infants below around two months of age. Doctors had to resort to using pediatric formulations designed for older children, thus exposing newborns to dangerous overdoses and toxicity, considering their immature liver function and unique metabolic needs (FT). Professor Umberto D'Alessandro of the London School of Hygiene & Tropical Medicine explained: "Neonates and young infants have compromised liver function and process certain drugs differently, so the dose employed in older children is not necessarily appropriate for wee babies."

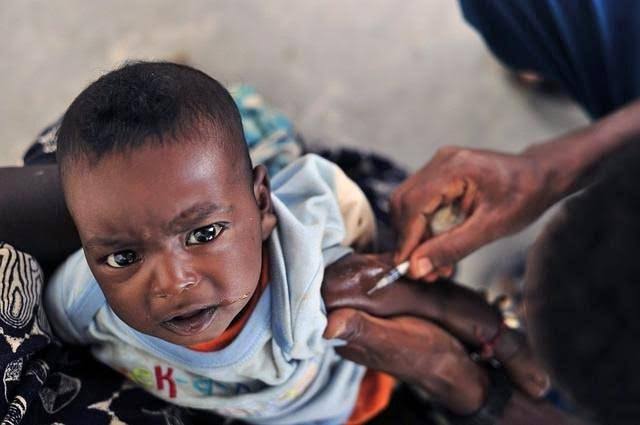
Fast Facts and Timeline: From Trials to Approval The clinical trials began in 2023, namely the CALINA study, recruiting even one-day-old infants to determine safe dosing in 2–5 kg infants (Biospace). On July 8, 2025, Swissmedic's approval was the result of an extensive evaluation of data, triggering a global health fast-track for anticipated widespread use in Africa. Eight countries in Africa, Burkina Faso, Côte d'Ivoire, Kenya, Malawi, Mozambique, Nigeria, Tanzania, and Uganda, will approve the drug within 90 days. Nigeria, the continent's most malaria-endemic country, is most likely to be among the early adopters.
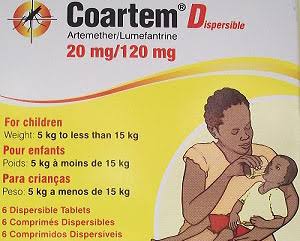
How the Drug Works: A Baby-Friendly Formulation Coartem Baby combines artemether-lumefantrine, the essence of today's Coartem treatment, but reengineered for neonates: I. Pleasant sweet cherry taste, easily dissolves in breast milk or water for convenient use II. Optimized dosage ratio to guard against toxicity; infants need to be shielded by mature enzyme systems III. Sold as Riamet Baby in some markets, it is modified from the trusted Coartem brand, yielding safe, infant-formulated treatment
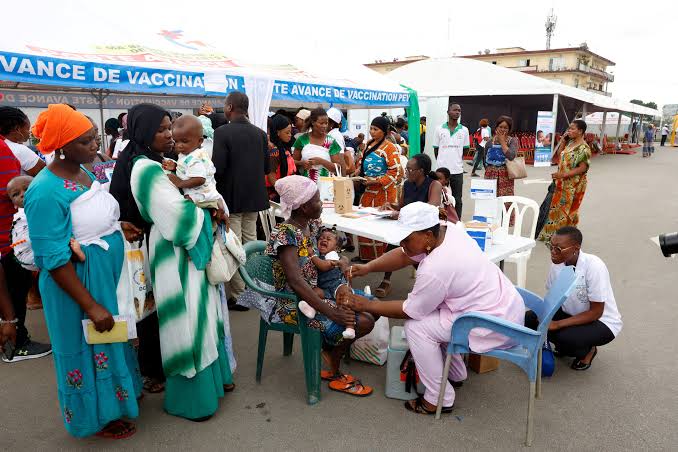
Big Picture: Malaria's Burden and Global Response Malaria remains a major killer: in 2023, there were 263 million cases and nearly 600,000 deaths, 75% of which were children under the age of five years, predominantly in sub-Saharan Africa (FT). Approximately 30 million babies are born yearly in malaria-endemic regions, and infants under six months of age have rates of infection ranging from 3.4%–18.4%. While malaria vaccines (RTS, S/Mosquirix and R21) are implemented for older infants, not neonates, yet again this highlights the urgent need for treatments like Coartem Baby.
Conclusion The approval of the registration of Coartem Baby fills a gap that is crucial for the prevention of malaria worldwide, shielding babies in their most vulnerable period. This innovation complements the implementation of vaccines and preventive interventions like bed nets, delivering comprehensive early-life malaria protection. As African authorities embark on approvals, nations like Nigeria, Kenya, and Tanzania will pave the way in implementation. The deployment is not merely about medical advancement but about a moral imperative: Every baby, however small, deserves lifesaving access to treatment.